The McAllister lab uses pre-clinical breast cancer models, patient biospecimens, and data from clinical studies to answer questions related to breast cancer progression and response to therapy. We are currently undertaking exciting projects in the following areas:
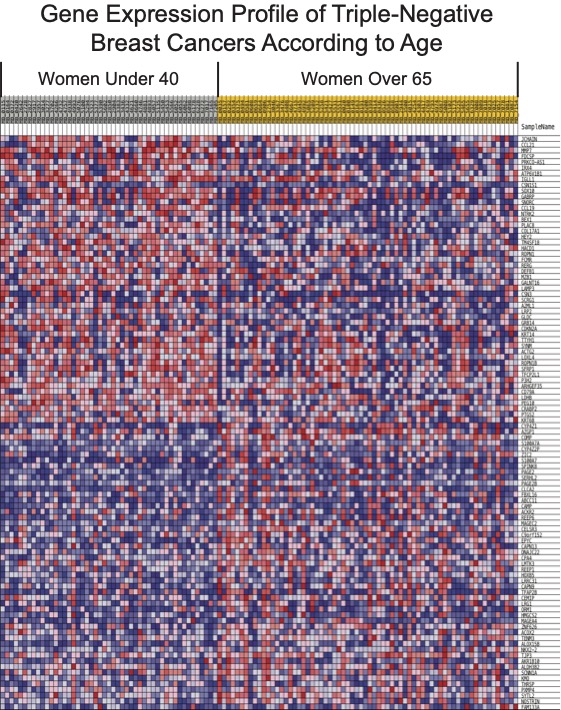
1. Understanding how immunologic age impacts breast cancer progression, therapeutic responses, and patient outcomes. Immunotherapy has been revolutionary for treating cancer patients. Nevertheless, immunotherapies are not effective for many breast cancer patients. Our efforts are directed at finding ways to improve treatment strategies. One way we are doing that is to consider patient age at the time of diagnosis and treatment. Immunotherapy is designed to unleash a patient’s immune system to attack cancer. We contend that age is an important consideration with respect to immunotherapy, given that normal aging is associated with profound changes to the immune system. However, because so few older patients are enrolled in clinical trials, we do not yet know whether immunotherapy will benefit patients of all age groups. In fact, most breast cancer patients enrolled in clinical trials are under the age of 60, yet most breast cancer patients are over the age of 60. Based on our earlier work to understand the effects of age on disease progression and response to immunotherapy, we launched clinical studies and formed the Older Women with Breast Cancer Research and Treatment Team. Our group is working to identify more effective treatment strategies by learning more about a patient’s immune status.
Marsh T, Wong I, Sceneay J, Barakat A, Qin Y, Sjödin A, Alspach E, Nilsson B, Stewart SA, McAllister SS. Hematopoietic Age at Onset of Triple-Negative Breast Cancer Dictates Disease Aggressiveness and Progression. Cancer Res. 2016 May 15;76(10):2932-43. PMID: 27197230; PMCID: PMC8504438.
Sceneay J, Goreczny GJ, Wilson K, Morrow S, DeCristo MJ, Ubellacker JM, Qin Y, Laszewski T, Stover DG, Barrera V, Hutchinson JN, Freedman RA, Mittendorf EA, McAllister SS. Interferon Signaling Is Diminished with Age and Is Associated with Immune Checkpoint Blockade Efficacy in Triple-Negative Breast Cancer. Cancer Discov. 2019 Sep;9(9):1208-27. PMID: 31217296.
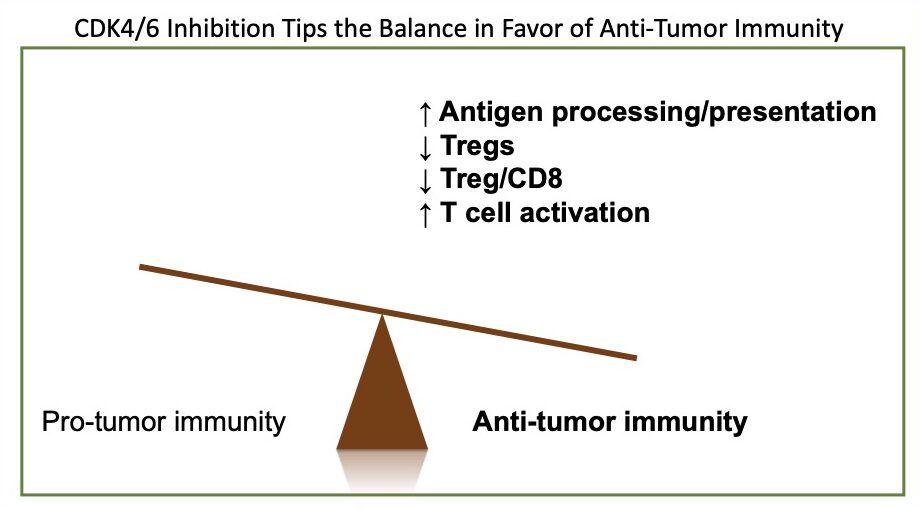
2. Understanding how CDK4/6 inhibitors can be used to enhance efficacy of immunotherapy. We conducted the first pre-clinical trial to evaluate CDK4/6 inhibitors combined with immune checkpoint blockade therapy (Goel, DeCristo, et al., Nature, 2017). That work formed the rationale for a clinical trial that reported improved objective response rates for breast cancer patients with metastatic hormone receptor-positive, HER2-negative disease. From those studies, we learned that CDK4/6 inhibitors trigger anti-tumor immunity. We are now working to understand better how CDK4/6 inhibitors impact the immune system. Ultimately, our goal is to design effective treatment strategies using CDK4/6 inhibitors with immunotherapy for patients with triple-negative breast cancer.
Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, Hoog J, Ellis MJ, Ma CX, Ramm S, Krop IE, Winer EP, Roberts TM, Kim HJ, McAllister SS, Zhao JJ. CDK4/6 Inhibition Triggers Anti-tumour Immunity. Nature. 2017 Aug 24;548(7668):471-475. PMID: 28813415; PMCID: PMC5570667.
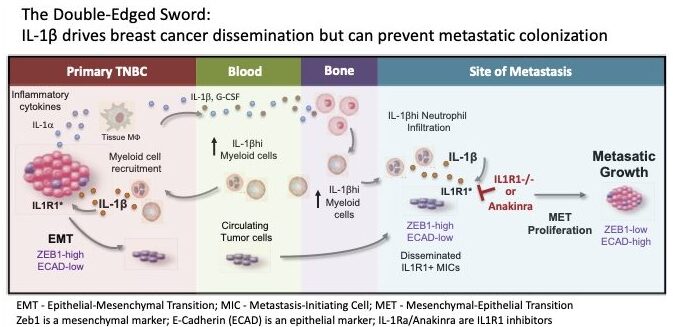
3. Understanding how hematopoietic and immune cells regulate metastasis. Our early work taught us that immune cells are recruited to breast tumors and their metastases. These immune cells can govern whether a cancer will advance or be halted. Our lab was among the first to understand that the hematopoietic progenitors of these cells in the bone marrow are already primed to support metastasis even when a primary breast tumor is in early stages. By studying and understanding how these bone marrow cells are different between cancer patients and women without cancer, we may be able to modulate these immune cells, or the molecules that they secrete, as a way to target metastases.
Castaño Z, San Juan BP, Spiegel A, Pant A, DeCristo MJ, Laszewski T, Ubellacker JM, Janssen SR, Dongre A, Reinhardt F, Henderson A, Del Rio AG, Gifford AM, Herbert ZT, Hutchinson JN, Weinberg RA, Chaffer CL, McAllister SS. IL-1β Inflammatory Response Driven by Primary Breast Cancer Prevents Metastasis-initiating Cell Colonization. Nat Cell Biol. 2018 Sep;20(9):1084-97. PMID: 30154549; PMCID: PMC6511979.
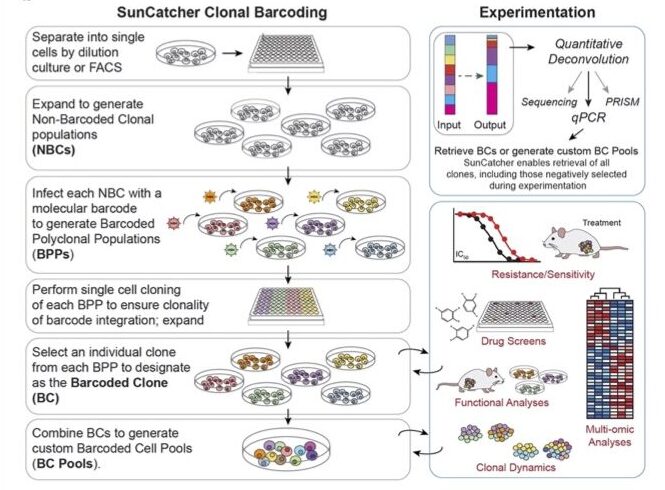
4. Development of SunCatcher, a new technology to accelerate research and discovery. We developed a clonal molecular barcoding method, which we term SunCatcher, that enables longitudinal tracking and retrieval of live barcoded cells for functional analysis.
Guo Q, Spasic M, Maynard A, Goreczny GJ, Olive JF, Bizuayehu A, McAllister SS. SunCatcher: Clonal Barcoding with qPCR–Based Detection Enables Functional Analysis of Live Cells and Generation of Custom Combinations of Cells for Research and Discovery. bioRxiv 2021 Oct;10.1101/2021.10.13.464251.
The team also developed a technology to accelerate discovery of new therapeutic strategies and biomarkers of response to therapy across many disease indications.
We invite you to contact us if you would like to learn more about our research.